

A neutrino is also involved in this process.
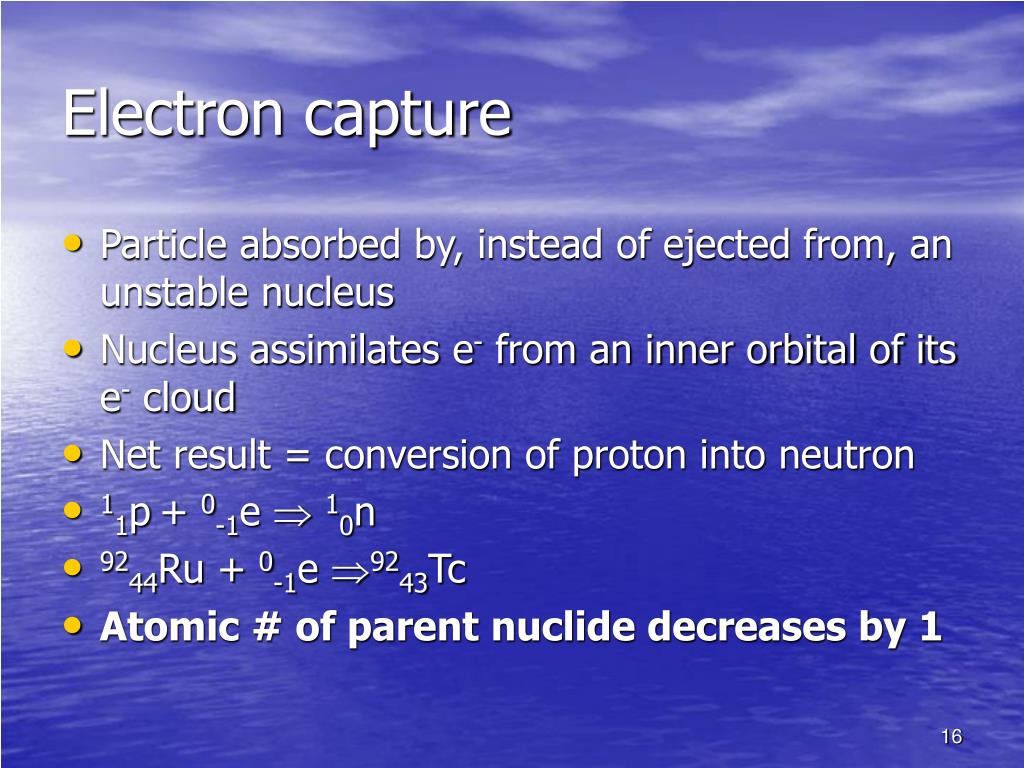
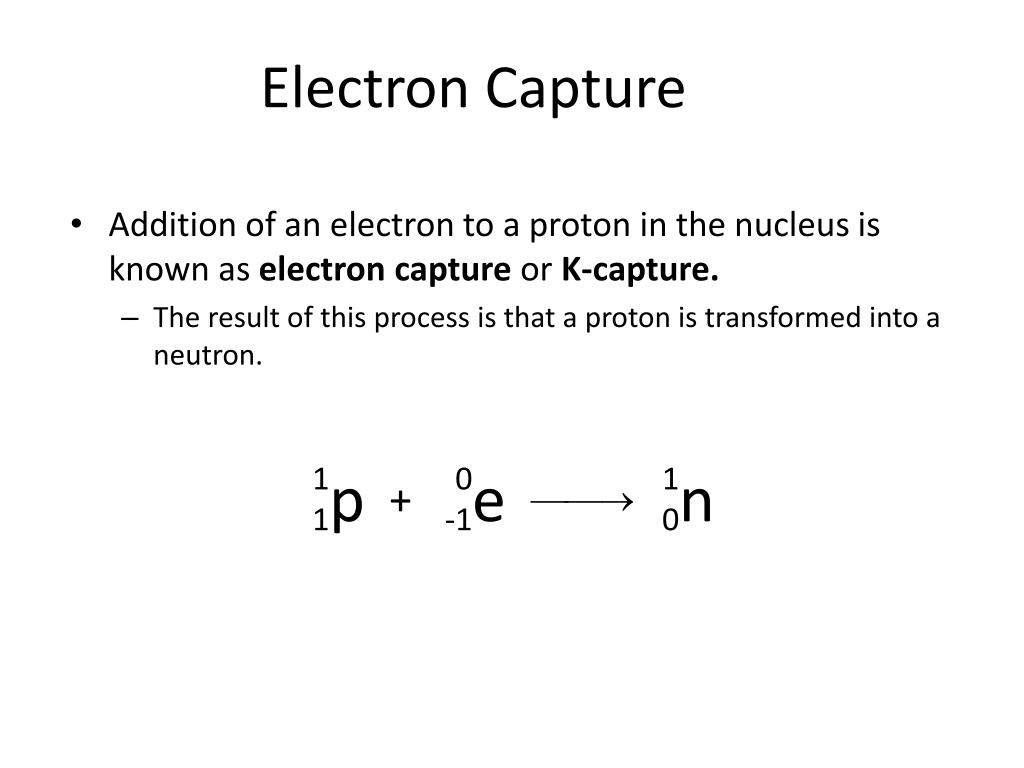 The electron must also be written on the left-hand side. The nuclide that decays is written on the left-hand side of the equation. The neutrino symbol is the Greek letter “nu.”Īn example of electron capture is shown below. The mass number and atomic number of the neutrino are zero. The general way of representing a positron emission is as above. The order of the nuclides on the right-hand side can be in any order. The nuclide that decays is the one on the left-hand side of the equation. What is the difference between Positron Emission and Electron Capture? Representation by an equation:Īn example of a positron emission (β + decay) is shown below. In addition, a lot of energy is released as gamma-rays. In this process, two things occur simultaneously a nuclear proton changes to a neutron after reacting with an electron which falls into the nucleus from one of its orbitals and the emission of an electron neutrino. This also results in nuclear transmutation, producing an atom of a chemical element into an element with an atomic number which is lower by one unit.Įlectron capture (also known as K-electron capture, K-capture, or L-electron capture, L-capture) involves absorption of an inner atomic electron, usually from its K or L electron shell by a proton-rich nucleus of an electrically neutral atom. Positron decay typically occurs in large ‘proton-rich’ radionuclides, because this process decreases the proton number relative to the neutron number. This process involves the conversion of a proton into a neutron inside a radionuclide nucleus while releasing a positron and an electron neutrino ( ν e).
The electron must also be written on the left-hand side. The nuclide that decays is written on the left-hand side of the equation. The neutrino symbol is the Greek letter “nu.”Īn example of electron capture is shown below. The mass number and atomic number of the neutrino are zero. The general way of representing a positron emission is as above. The order of the nuclides on the right-hand side can be in any order. The nuclide that decays is the one on the left-hand side of the equation. What is the difference between Positron Emission and Electron Capture? Representation by an equation:Īn example of a positron emission (β + decay) is shown below. In addition, a lot of energy is released as gamma-rays. In this process, two things occur simultaneously a nuclear proton changes to a neutron after reacting with an electron which falls into the nucleus from one of its orbitals and the emission of an electron neutrino. This also results in nuclear transmutation, producing an atom of a chemical element into an element with an atomic number which is lower by one unit.Įlectron capture (also known as K-electron capture, K-capture, or L-electron capture, L-capture) involves absorption of an inner atomic electron, usually from its K or L electron shell by a proton-rich nucleus of an electrically neutral atom. Positron decay typically occurs in large ‘proton-rich’ radionuclides, because this process decreases the proton number relative to the neutron number. This process involves the conversion of a proton into a neutron inside a radionuclide nucleus while releasing a positron and an electron neutrino ( ν e). ELECTRON CAPTURE CHEMISTRY PLUS
Positron emission is a type of radioactive decay and a sub-type of beta decay and is also known as beta plus decay ( β + decay). This is the key difference between positron emission and electron capture. In electron capture, the unstable nucleus captures one of the electrons from one of its orbitals and then produces a neutron. In positron emission, a positron (opposite of an electron) is also created in addition to the neutron. To solve this problem, these processes result in changing a proton in the nucleus into a neutron but in two different ways. Both these radioactive processes occur in unstable nuclei where there are too many protons and fewer neutrons. Although they result in changes in the nucleus, these two processes take place in two different ways. Positron emission and electron capture and are two types of nuclear processes. Key Difference – Positron Emission vs Electron Capture







 0 kommentar(er)
0 kommentar(er)
